Old T2000, New Genesis Processor: How to Perform Validation?
Elisabete Pereira Palhas, Biomedical Scientist, Diagnostic Cytology, Royal Glamorgan Hospital Llantrisant, Wales
In cytology, a variety of methods are available when talking about slide preparation, ranging from cytospin, megafunnel, smear, and liquid-based cytology (LBC). In the UK, two main companies provide LBC processors: BD SurePath and Hologic ThinPrep. At the Royal Glamorgan Hospital, ThinPrep has been our trusted choice for years when preparing slides for Papanicolaou staining.
As many of us know, the Cytyc ThinPrep 2000 is coming to the end of its service life, and a modern version, the Genesis, is taking its place. As a biomedical scientist supporting diagnostic cytology, I was given the responsibility of validating the new Genesis processor. As it was my first time validating brand-new equipment in the laboratory, I was a bit nervous and unfamiliar with the whole process, but with some guidance, determination and ambition to learn, I managed to complete the validation. For the laboratories still using the T2000; the good news is that the Genesis operates in a very similar but simpler way, a win-win situation.

The results obtained from the slides prepared on the Hologic Genesis Processor are expected to be the same or of better quality when compared to the current ThinPrep 2000 processor.
As stated in the manufacturer’s manual, Hologic Genesis Processor is expected to complete the sample preparation process successfully:

In terms of sensitivity and specificity, the manufacturer states that in a study conducted, no statistically significant differences were found in performance between the ThinPrep Genesis and the ThinPrep 2000 system. Their results are shown in the table below.
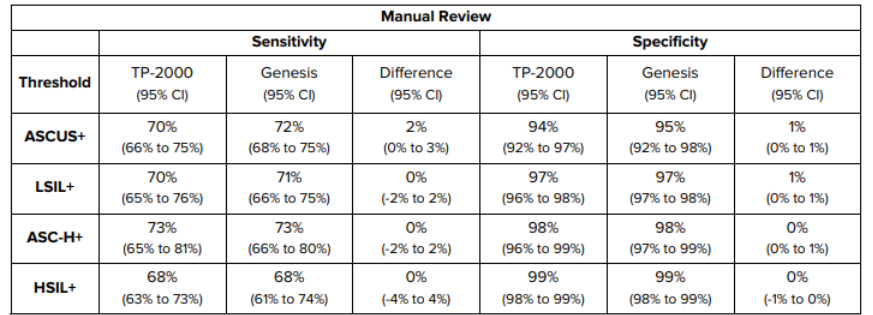
Table 1: Performance Summary – ThinPrep Genesis Processor Results vs ThinPrep 2000 System Results for Slides with Manual Review (Sensitivity and Specificity).
The manufacturer also evaluates cellular carry-over, migration of cells from one sample/patient to the following one, and compares between the two processors: ThinPrep 2000 System and ThinPrep Genesis Processor. 350 abnormal samples were processed, alternating with 350 Preservcyt vials with no cells. Once processed, and stained, the slides were reviewed by cytotechnologists. One slide was excluded in the ThinPrep 2000 System due to operator error and the criteria was that slides containing at least one cell were considered positive for cellular cross-contamination. The results shown in the table below demonstrate that the cellular carry-over from slide to slide on the ThinPrep Genesis is not inferior to the ThinPrep 2000 System.
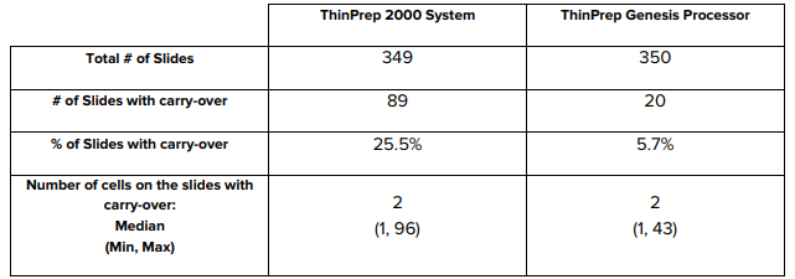
Table 2: Cellular Carry-Over.
For our validation, a representative selection of cytology specimen types, including EBUS, bronchial washings, serous fluids, and urine was chosen, and two slides from the same Preservcyt solution vial were processed, the first slide to be processed on the T2000 and the second slide to be processed on the Genesis. This is included as a measure of uncertainty, because we conditioned the selection of cells present in the second slide. It was agreed this method was better than splitting the original sample into two different Preservcyt vials due to the way our laboratory processes specimens. However, it is advisable each laboratory must select the best fitting method for their processes.
Unfortunately, the slides were showing a double rim effect created by a defect on the instrument.
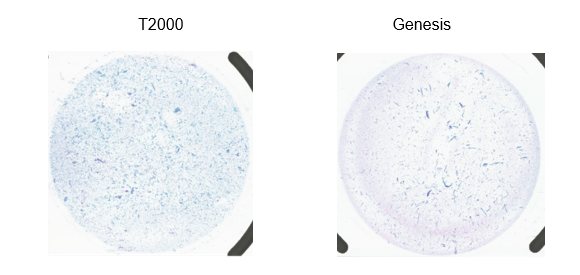
I reached out to the BAC team for help, to check whether this was a common and quick issue to fix. The team did their best to help me by contacting other laboratories that already use this processor. It turned out the problem was not that easy to repair, so I contacted Hologic’s technical support team. They sent a third-party consultant Hologic supporter to help us on site. Upon his lovely visit, he raised the defect with the engineer and also suggested staining improvement. We took the advice into consideration. The engineer later identified and fixed the problem, a loose screw on the slide positioning area creating imbalances when pressed to the filter.
Following the engineer's visit, the instrument was fixed, the staining protocol improved, and a second run of slides was made for our in house validation process, following UK NEQAS CPT Diagnostic Cytology quality assessment criteria to assess the slides:
Slides reviewed and accepted by a pathologist.
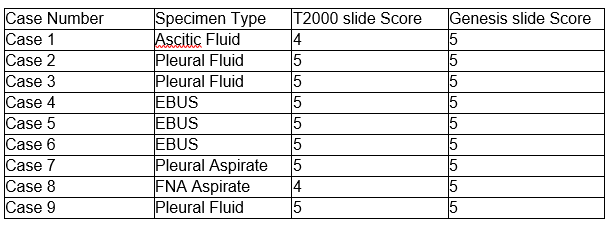
When comparing the scores between the T2000 slide and Genesis slide the statistical concordance obtained was 0.9. As seen in the table above, the scores match in the majority of the cases except for Case 1 and Case 8 where the scores from the Genesis were higher compared to the slides from the T2000.
For internal quality control, the instrument performs a Power On Self Test (POST). When the Genesis is powered on, the electrical, software and mechanical systems are aligned and tested to confirm its performance.
Advantages:
- Unlike the ThinPrep 2000 Processor, the ThinPrep vials are placed closed in the new Genesis Processor. The major advantage is the minimal exposure to the sample.
- User-friendly. Only the first slide preparation requires the adequate set-up, after that, each slide is automatically processed as soon as the door is closed and instrument detects the presence of the filter, the Preservcyt pot and the fixation vial.
Disadvantages:
- Our laboratory does not utilise the full instrument potential, as the Genesis can perform both slide and aliquot (good for molecular). However, because it requires an additional purchase, our laboratory did not acquire it, so we only perform slide preparation.
-Opposite slide orientation compared with the T2000.
Slide orientation in the Genesis is facing down and left and in the T2000 was facing down and right. In the beginning, it can be tricky for the members of staff as they are already used to the way T2000 operates. However, this step will be overcome with time and practice.
Genesis slide loading, with the frosted area to the left and facing down.
In terms of maintenance, it is important to check at the beginning of each day if the fixative level is within the normal range or if it needs to be either replaced or topped up. Wipe the slide nest and grippers to remove any glass dust or debris. At the end of each slide processing, I would suggest cleaning the filter plug and filter puncture point with a cloth soaked in 70% alcohol to remove any Preservcyt solution remaining.
Tips:
- This instrument can easily generate reports helpful to record error logs and data tracking.
- The processor tends to heat up quick easily. Suggestion: leave the door open when not in use.
- Turn off the power at the end of each working day for the instrument to do a daily POST.
- Suggestion for set up from day one:
- Sound level 1 - the instrument is already loud,
- Select different alert tones for errors and processing complete – staff can easily identify the status by the sound.
Auto-start with Close Door On – less steps to go through as it starts immediately if all components inside.
Conclusion
On my first experience of instrument validation, I would say that it was both challenging and exciting. It took longer than expected due to instrument defects along the way, as well as knowing whom to contact for help and how to proceed. For this, I want to thank the BAC team, especially Donna, for your support. I truly felt heard and helped by the community!
Staff training on the Genesis is a work in progress as they get rotated into the diagnostic cytology lab.
Troubleshooting skills, experience and confidence will grow over time.
References:
Hologic, 2023. ThinPrep™ Genesis™ Processor Operator’s Manual. Available at: ThinPrep Genesis processor | Hologic
UK NEQAS, 2025. Assessment Criteria Handbook Diagnostic Cytopathology. Edition 11.
Images from ‘Hologic, 2023. ThinPrep™ Genesis™ Processor Operator’s Manual’ used with kind permission from Hologic.
